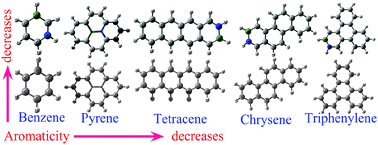
We have studied the topological and local aromaticity of BN-substituted benzene, pyrene, chrysene, triphenylene and tetracene molecules. The nucleus-independent chemical shielding (NICS), harmonic oscillator model of aromaticity (HOMA), para-delocalization index (PDI) and aromatic fluctuation index (FLU) have been calculated to quantify aromaticity in terms of magnetic and structural criteria. We find that charge separations due to the introduction of heteroatoms largely affect both the local and topological aromaticity of these molecules. Our studies show that the presence of any kind of heteroatom in the ring not only reduces the local delocalization in the six membered ring, but also affects strongly the topological aromaticity. In fact, the relative orders of the topological and local aromaticity depend strongly on the position of the heteroatoms in the structure. In general, more ring shared BN containing molecules are less aromatic than the less ring shared BN molecules. In addition our results provide evidence that the structural stability of the molecule is dominated by the σ bond rather than the π bond.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...