Interaction of hydrogen with surfaces of silicates: single crystal vs. amorphous†
Abstract
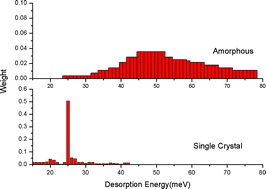
We have studied how the formation of molecular hydrogen on silicates at low temperature is influenced by surface morphology. At low temperature (<30 K), the formation of molecular hydrogen occurs chiefly through weak physical

 Please wait while we load your content...
Please wait while we load your content...