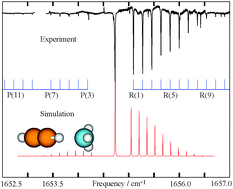
We report a combined high resolution infrared and microwave spectroscopic investigation of the acetylene–ammonia and carbonyl sulfide–ammonia complexes using a pulsed slit-nozzle multipass absorption spectrometer based on a quantum cascade laser and a pulsed nozzle beam Fourier transform microwave spectrometer, respectively. The ro-vibrational transitions of the acetylene–ammonia complex have been measured at 6 μm in the vicinity of the ν4 band of ammonia for the first time. The previously reported pure rotational transitions have been extended to higher J and K values with 14N nuclear quadrupole hyperfine components detected and analyzed. The spectral analysis reveals that acetylene binds to ammonia through a C–H⋯N weak hydrogen bond to form a C3v symmetric top, consistent with the previous microwave [Fraser et al., J. Chem. Phys., 1984, 80, 1423] and infrared spectroscopic study at 3 μm [Hilpert et al., J. Chem. Phys., 1996, 105, 6183]. A parallel study has also been carried out for the carbonyl sulfide–ammonia complex whose pure rotational and ro-vibrational spectra at 6 μm have been detected and analyzed for the first time. The spectral and the subsequent structural analyses, in conjunction with the corresponding ab initio calculation, indicate that the OCS–NH3 complex assumes C3v symmetry with S pointing to N of NH3, in contrast to the T-shaped geometries obtained for the isoelectronic N2O–NH3 and CO2–NH3 complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...