On the dimerization of chlorophyll in photosystem II†
Abstract
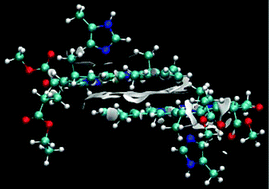
In photosystem II, absorbed light energy is transferred to a reaction centre consisting of chlorophyll units. Release of an electron from the reaction centre is the starting point for the charge separation and electron transport chain in PSII. Crystal structures of the reaction centre have identified two

 Please wait while we load your content...
Please wait while we load your content...