Possible mechanism underlying high-pressure unfolding of proteins: formation of a short-period high-density hydration shell
Abstract
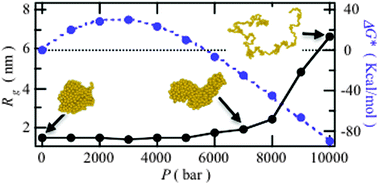
Hydration effects on high-pressure unfolding of a hydrophobic polymer chain are investigated through a multiscale simulation based on density-functional theory. The results strongly suggest the following: a thermodynamic origin for high-pressure denaturation, i.e., the decrease in volume due to the unfolding can be explained by the formation of a short-period high-density hydration shell.

 Please wait while we load your content...
Please wait while we load your content...