Theoretical study on the gas phase reaction of acrylonitrile with a hydroxyl radical†
Abstract
The mechanism and kinetics of the reaction of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCN) with
CHCN) with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond or C atom of –CN group to form the chemically activated adducts, 1-IM1(HOCH2
C double bond or C atom of –CN group to form the chemically activated adducts, 1-IM1(HOCH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCN), 2-IM1(CH2
CHCN), 2-IM1(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) HOCHCN), and 3-IM1(CH2
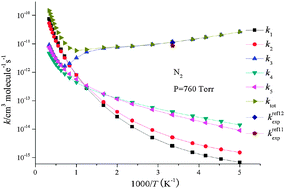
HOCHCN), and 3-IM1(CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOHN) via low barriers, and direct hydrogen abstraction paths may also occur. Temperature- and pressure-dependent rate constants have been evaluated using the Rice–Ramsperger–Kassel–Marcus theory. The calculated rate constants are in good agreement with the experimental data. At atmospheric pressure with N2 as bath gas, 1-IM1(OHCH2
CHCOHN) via low barriers, and direct hydrogen abstraction paths may also occur. Temperature- and pressure-dependent rate constants have been evaluated using the Rice–Ramsperger–Kassel–Marcus theory. The calculated rate constants are in good agreement with the experimental data. At atmospheric pressure with N2 as bath gas, 1-IM1(OHCH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCN) formed by collisional stabilization is the major product in the temperature range of 200–1200 K. The production of CH2CCN and CHCHCNviahydrogen abstractions becomes dominant at high temperatures (1200–3000 K).
CHCN) formed by collisional stabilization is the major product in the temperature range of 200–1200 K. The production of CH2CCN and CHCHCNviahydrogen abstractions becomes dominant at high temperatures (1200–3000 K).

 Please wait while we load your content...
Please wait while we load your content...