Stereocontrolled (3+2) cycloadditions between azomethine ylides and dipolarophiles: a fruitful interplay between theory and experiment
Abstract
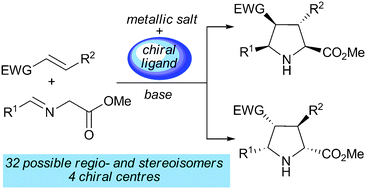
In this article we present recent developments in (3+2) cycloadditions with special emphasis on 1,3-dipolar reactions involving

 Please wait while we load your content...
Please wait while we load your content...