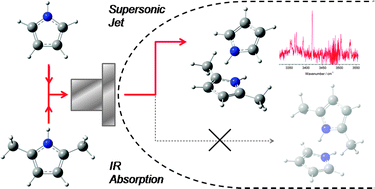
N–H⋯π hydrogen-bonded (H-bonded) structures were studied by applying vibrational spectroscopy to self-aggregate clusters of 2,5-dimethylpyrrole (DMPy) and its binary clusters with pyrrole (Py). The NH stretching vibrations of jet-cooled clusters were observed by IR cavity ringdown spectroscopy. A combination of experiments and density functional theory calculations revealed the stable structures, intermolecular binding energies, and harmonic vibrational frequencies. The IR spectrum of the DMPy self-aggregate clusters was very similar in spectral features to that of the Py clusters in a previous work. The observed NH stretching vibrations at 3505, 3420, 3371, and 3353 cm−1 are simultaneously red-shifted by ∼25 cm−1 from the Py monomer, dimer, trimer, and tetramer, respectively. Based on a spectral analogy of DMPy with Py, and a consistency of the calculated harmonic frequencies with experiments, the H-bonded structures of the DMPy clusters were determined to be of a T-shape for a dimer and a cyclic for a trimer and a tetramer. For the DMPy–Py binary clusters, we discussed the stability and geometry of the N–H⋯π interactions in the T-shaped dimer and the cyclic trimer. The binary dimer showed the only single NH stretch at 3419 cm−1 in the IR spectrum. A vibrational analysis of the H-bonded NH stretches as well as the calculated stabilization energies deduced that only the binary dimer by DMPy as an acceptor and Py as a donor can exist in a supersonic jet. For binary trimers, NH stretches were observed due to both (DMPy)2–(Py)1 and (DMPy)1–(Py)2. They were found to have different vibrational patterns from each other; the former showed three dispersed NH stretches, and the other had two quasi-degenerate NH stretches. Throughout this study, we also considered the intermolecular geometries, such as the H-bond distance and the angle in terms of the methyl group substitution effect.
 Please wait while we load your content...
Please wait while we load your content...