Nature of anion-templated π+–π+ interactions†
Abstract
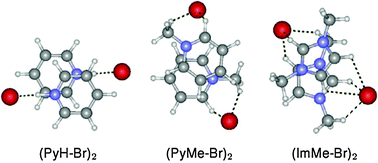
Interaction between positively charged aromatic groups (π+–π+) is characterized by anti-parallel, displaced-stacked structures in the presence of counteranions. Binding energies of pyridinium, N-methylpyridinium and N-methylimidazolium dimers are much larger than that of

 Please wait while we load your content...
Please wait while we load your content...