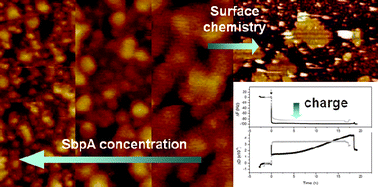
Bacterial crystalline surface layers (S-layers) are the outermost envelope of prokaryotic organisms representing the simplest biological membranes developed during evolution. In this context, the bacterial protein SbpA has already shown its intrinsic ability to reassemble on different substrates forming protein crystals of square lattice symmetry. In this work, we present the interaction between the bacterial protein SbpA and five self-assembled monolayers carrying methyl (CH3), hydroxyl (OH), carboxylic acid (COOH) and mannose (C6H12O6) as functional groups. Protein adsorption and S-layer formation have been characterized by atomic force microscopy (AFM) while protein adsorption kinetics, mass uptake and the protein layer viscoelastic properties were investigated with quartz crystal microbalance with dissipation monitoring (QCM-D). The results indicate that the protein adsorption rate and crystalline domain area depend on surface chemistry and protein concentration. Furthermore, electrostatic interactions tune different protein rate adsorption and S-layer recrystallization pathways. Electrostatic interactions induce faster adsorption rate than hydrophobic or hydrophilic interactions. Finally, the shear modulus and the viscosity of the recrystallized S-layer on CH3C6S, CH3C11S and COOHC11S substrates were calculated from QCM-D measurements. Protein–protein interactions seem to play a main role in the mechanical stability of the formed protein (crystal) bilayer.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...