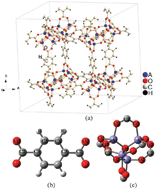
Formation energies, chemical bonding, electronic structure, and optical properties of metal–organic frameworks of alkaline earth metals, A–IRMOF-1 (where A = Be, Mg, Ca, Sr, or Ba), have been systemically investigated with DFT methods. The unit cell volumes and atomic positions were fully optimized with the Perdew–Burke–Ernzerhof functional. By fitting the E–V data into the Murnaghan, Birch and Universal equation of states (UEOS), the bulk modulus and its pressure derivative were estimated and provided almost identical results. The data indicate that the A–IRMOF-1 series are soft materials. The estimated bandgap values are all ca. 3.5 eV, indicating a nonmetallic behavior which is essentially metal independent within this A–IRMOF-1 series. The calculated formation energies for the A–IRMOF-1 series are −61.69 (Be), −62.53 (Mg), −66.56 (Ca), −65.34 (Sr), and −64.12 (Ba) kJ mol−1 and are substantially more negative than that of Zn-based IRMOF-1 (MOF-5) at −46.02 kJ mol−1. From the thermodynamic point of view, the A–IRMOF-1 compounds are therefore even more stable than the well-known MOF-5. The linear optical properties of the A–IRMOF-1 series were systematically investigated. The detailed analysis of chemical bonding in the A–IRMOF-1 series reveals the nature of the A–O, O–C, H–C, and C–C bonds, i.e., A–O is a mainly ionic interaction with a metal dependent degree of covalency. The O–C, H–C, and C–C bonding interactions are as anticipated mainly covalent in character. Furthermore it is found that the geometry and electronic structures of the presently considered MOFs are not very sensitive to the k-point mesh involved in the calculations. Importantly, this suggests that sampling with Γ-point only will give reliable structural properties for MOFs. Thus, computational simulations should be readily extended to even more complicated MOF systems.

 Please wait while we load your content...
Please wait while we load your content...