On the dissociation of molecular hydrogen by Au supported on transition metal carbides: choice of the most active support
Abstract
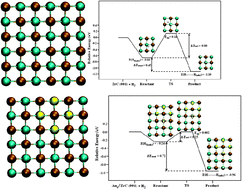
A systematic density functional study of the adsorption and dissociation of H2 on the clean (001) surface of various transition metal carbides (TMCs; TM = Ti, Zr, V, Mo) and on Au4 nanoclusters supported on these TMCs is presented. It is found that the H2 dissociation on the bare clean TMCs strongly depends on the chemical nature of the support. Thus, the H2 molecule interacts rather strongly with TiC(001) and ZrC(001) but very weakly with VC(001) and δ-MoC(001). For the supported Au4 cluster, two different types of molecular mechanisms are found. For Au4/TiC(001) and Au4/ZrC(001), H2 dissociation leads to a H atom directly interacting with the Au4 cluster while the second H atom is transferred to the support. In contrast, for Au4/VC(001) and Au4/δ-MoC(001), both H atoms interact with the Au4 cluster. Overall, the present study suggests that, among the systems studied, Au/ZrC is the best substrate for H2 dissociation.

 Please wait while we load your content...
Please wait while we load your content...