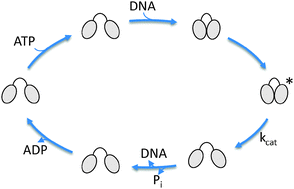
Reverse gyrase introduces positive supercoils into DNA in an ATP-dependent process. It has a modular structure comprising a helicase-like and a topoisomerase domain. The helicase-like domain consists of two RecA-like subdomains and thus structurally resembles members of the helicase superfamily 2. It is a nucleotide-dependent switch that alters between an ATP state with a slight preference for dsDNA, and an ADP state with a high preference for ssDNA. Inter-domain communication between the helicase-like and the topoisomerase domain is central for their functional cooperation in reverse gyrase. The latch, an insertion into the helicase-like domain, has been suggested as an important element in coordinating their activities. Here, we have dissected the nucleotide cycle of the reverse gyrase helicase-like domain in the absence and presence of different DNA substrates. With this comprehensive thermodynamic characterization of the nucleotide cycle of the helicase-like domain, in combination with single molecule FRET data on the conformation of the helicase-like domain at all stages of the catalytic cycle, a picture emerges as to how the helicase-like domain may guide ATP-dependent positive supercoiling by reverse gyrase.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...