Spectral interpretation of thermally irreversible recovery of poly(N-isopropylacrylamide-co-acrylic acid) hydrogel†
Abstract
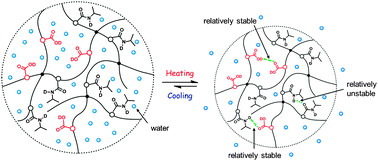
The thermally induced volume phase transition process of poly(N-isopropylacrylamide-co-acrylic acid) (PNIPAM-co-AA) hydrogel is studied using

 Please wait while we load your content...
Please wait while we load your content...