One-pot generation of mesoporous carbon supported nanocrystalline calcium oxides capable of efficient CO2 capture over a wide range of temperatures†
Abstract
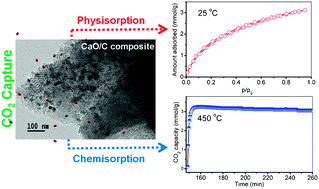
Ordered mesoporous carbon-supported
- This article is part of the themed collection: Controlled nanostructures for applications in catalysis

 Please wait while we load your content...
Please wait while we load your content...