Should negative electron affinities be used for evaluating the chemical hardness?
Abstract
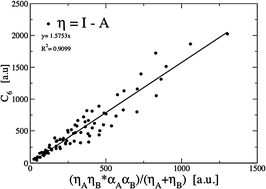
Despite recent advances in computing negative electron affinities using density-functional theory, it is an open issue as to whether it is appropriate to use negative electron affinities, instead of zero electron affinity, to compute the chemical hardness of atoms and molecules with metastable anions. We seek to answer this question using the accepted empirical rules linking the chemical hardness to the atomic size and the polarizability; we also propose a new correlation with the C6 London dispersion coefficient. For chemical reactivity in the gas phase, it seems to make no difference whether negative, or zero, electron affinities are used for systems with metastable anions. For reactions in solution the evidence that is presently available is insufficient to establish a preference. In addressing this issue, we noted that electron affinity data from which atomic chemical hardness values are computed are out of date; an update to Pearson's classic 1988 table [Inorg. Chem., 1988, 27, 734–740] is thus provided.

 Please wait while we load your content...
Please wait while we load your content...