Conformational analysis and UV/Vis spectroscopic properties of a rotaxane-based molecular machine in acetonitrile dilute solution: when simulations meet experiments†
Abstract
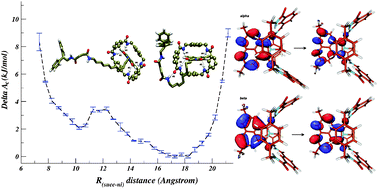
A reduced form of a synthetic hydrogen-assembled molecular shuttle for nano-technological applications has been investigated by molecular dynamics simulations and density functional theory calculations. It is composed by a benzylic amide ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNR hydrogen bonds. Simulations also revealed that the geometry of the supramolecular assembly reversibly oscillates between unfolded and folded conformations, with the latter characterized by an electrostatic hook involving the succinamide end group and the
CNR hydrogen bonds. Simulations also revealed that the geometry of the supramolecular assembly reversibly oscillates between unfolded and folded conformations, with the latter characterized by an electrostatic hook involving the succinamide end group and the

 Please wait while we load your content...
Please wait while we load your content...