Electronic structure calculations of low-lying electronic states of O3
Abstract
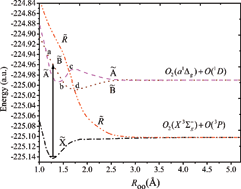
Configuration-based multi-reference second order perturbation theory (CB-MRPT2) and multi-reference configuration interaction with single and double excitations (MRCISD) have been used to calculate the bending and dissociation potential energy curves (PECs) of ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 1A1 are determined by reference-selected MRCISD. Furthermore, one-dimensional cuts along the dissociation reaction coordinate for the lowest four electronic states of O3 with 1A′ symmetry and possible pre-dissociations are studied. The Hartley band may be pre-dissociable, and the pre-dissociation limit is found to be 3871 cm−1, which corresponds to symmetric stretching quanta nss ≈ 6.
1A1 are determined by reference-selected MRCISD. Furthermore, one-dimensional cuts along the dissociation reaction coordinate for the lowest four electronic states of O3 with 1A′ symmetry and possible pre-dissociations are studied. The Hartley band may be pre-dissociable, and the pre-dissociation limit is found to be 3871 cm−1, which corresponds to symmetric stretching quanta nss ≈ 6.

 Please wait while we load your content...
Please wait while we load your content...