Local defects enhanced dehydrogenation kinetics of the NaBH4-added Li–Mg–N–H system
Abstract
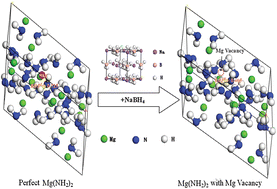
A mechanistic understanding on the enhanced kinetics of hydrogen storage in the NaBH4-added Mg(NH2)2-2LiH system is provided by carrying out experimental investigations associated with first-principles calculations. It is found that the operating temperatures for hydrogen desorption of the Mg(NH2)2-2LiH system are reduced by introducing NaBH4, and the NaBH4 species seems almost unchanged during dehydrogenation/

 Please wait while we load your content...
Please wait while we load your content...