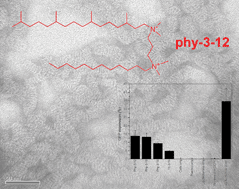
Synthesis and aggregation properties of dissymmetric phytanyl-gemini surfactants for use as improved DNA transfection vectors
Abstract
Improvements in transfection efficiency are required in order to make the goal of cellular gene delivery by non-viral vectors realizable. Novel derivatives of gemini

 Please wait while we load your content...
Please wait while we load your content...