Molecules with hypercoordinate planar centers have continued to receive enthusiastic attention due to their violation of the traditional models of three-dimensional chemical bonding and maximum tetracoordination. These electronic exotic but structurally aesthetic species have been optimistically conceived as building blocks in cluster-assembly for bulky materials. Recently, the planar hexacoordinate nitrogen (phN) unit, NB6−, has been theoretically incorporated into a series of sandwich-like transition-metal compounds. However, the intrinsic stability of NB6− in both gas-phase and assembly has not been tackled, though it is the key factor for predicting the viability of any molecules. In this paper, at the CCSD(T)/6-311+G(2df)//B3LYP/6-311+G(d)+ZPVE level, we investigate for the first time the thermodynamic and kinetic stability of the phN unit, NB6−, in both free and assembled ([NB6]2Fe) forms. The calculated least barrier height of phN towards conversion is 9.2 and 4.4 kcal mol−1 in free and assembled forms, respectively. Most importantly, the phN structure is thermodynamically rather unstable, by 102.8 and 162.1 kcal mol−1 higher than the respective lower-lying conversion isomers. Therefore, in view of the combined thermodynamic and kinetic consideration, we propose that isolation of the phN structure of NB6− in either gas phase or assembly is unlikely. The present results manifest that for predicting any viable molecule with exotic structures, investigation of its “intrinsic stability” is highly necessary. The maintenance of the phN–NB6− is discussed at the 6-311+G(d)-B3LYP, MP2, CCSD and CCSD(T) optimization levels in comparison with the isoelectronic and milestone phC–CB62−.
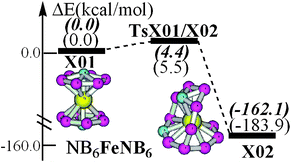
 Please wait while we load your content...
Please wait while we load your content...