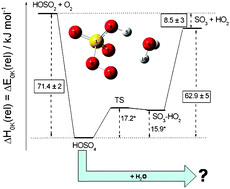
A detailed theoretical analysis of the HOSO2 + O2 reaction is performed, paying special attention to the kinetics of the intermediate HSO5 radical. The possible formation of the monohydrated adduct, HSO5·H2O, in the presence of water vapor is examined. For the binding energy of the most stable isomer at T = 0 K, a value of D0(HOSO4–H2O) = 51.7 kJ mol−1 was obtained at the CCSD(T)/CBS level of theory; other energies were adopted from a recently published high-level quantum chemical study of our laboratory. Molecular geometries and vibrational frequencies of the reactants, intermediates, and products were obtained from B3LYP/cc-pVTZ calculations. Rate coefficients and lifetimes of the HSO5 intermediate were calculated by solving a master equation with specific rate coefficients from statistical rate theory. The master equation is extended by a bimolecular sink term, which accounts for the HSO5 + H2O reaction. The relation between thermal and chemical activation in this reaction system is examined. The results show that the lifetime of the HSO5 intermediate under atmospheric conditions is too short for a bimolecular reaction with water to become important. Relative yields of HSO5·H2O well below 1% were obtained, ruling out this reaction pathway as a relevant source for aerosols.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...