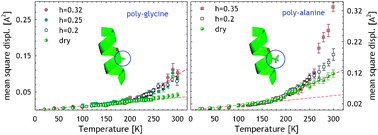
Two main onsets of anharmonicity are present in protein dynamics. Neutron scattering on protein hydrated powders revealed a first onset at about 150 K and a second one at about 230 K (the so called dynamical transition). In order to assess the molecular origin of protein anharmonicity, we study different homomeric polypeptides by incoherent elastic neutron scattering, thus disentangling the contribution of different molecular groups in proteins. We show that methyl group rotations are the main contributors to the low temperature onset. Concerning the dynamical transition, we show that it also occurs in absence of side chains; however, the presence and mobility of side chains substantially increases the fluctuations amplitude without influencing the transition temperature. We also investigate the role of hydration on the anharmonic contributions. Our study shows that methyl group rotations are unaffected by hydration and confirms that the dynamical transition is suppressed in dry samples. In hydrated samples, while the pure backbone contribution does not depend on the hydration h at h ≥ 0.2, in the presence of side chains the anharmonic fluctuations involved in the dynamical transition are enhanced by increasing the water content.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...