Systematic microcanonical analyses of polymer adsorption transitions
Abstract
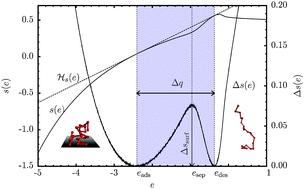
In detailed microcanonical analyses of densities of states obtained by extensive multicanonical Monte Carlo computer simulations, we investigate the caloric properties of conformational transitions that adsorbing

 Please wait while we load your content...
Please wait while we load your content...