The influence of the relative position of the thiophene and pyrrole rings in donor–acceptor thienylpyrrolyl-benzothiazole derivatives. A photophysical and theoretical investigation
Abstract
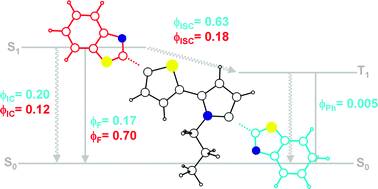
A detailed spectroscopic and photophysical study has been carried out on a series of

 Please wait while we load your content...
Please wait while we load your content...