Application of the additivity of group energies to understand conformational preference: the anomeric effect†
Abstract
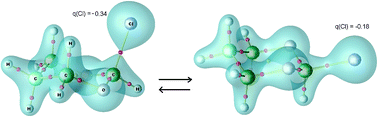
The conformational preference in normal and reverse anomeric effects is analyzed by taking advantage of the known additivity and transferability of functional
 Please wait while we load your content...
Please wait while we load your content...