Enhancement of structural and charge-transfer barrier properties of n-alkanethiol layers on a polycrystalline copper surface by electrochemical potentiodynamic polarization
Abstract
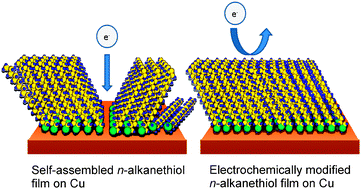
A method that significantly improves the charge-transfer barrier properties of self-assembled

 Please wait while we load your content...
Please wait while we load your content...