Is an elementary reaction step really elementary? Theoretical decomposition of asynchronous concerted mechanisms
Abstract
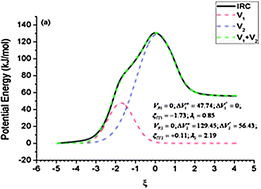
In this paper the primitive process concept is used to reproduce and explain the potential energy profile of an asynchronous concerted mechanism. The number of primitive processes constituting such a mechanism can be deduced from the study of the influence of the number of electrons on the potential energy profile of the elementary step. The study of a particular example indicates that the position of the transition states of the primitive processes constituting an asynchronous concerted mechanism seems not to be affected by the number of electrons in the system. This implies that the maximum hardness and minimum electrophilicity principles are valid for primitive processes but not for asynchronous concerted mechanisms. It appears that a potential energy profile with a shoulder before the transition state, and a reaction

 Please wait while we load your content...
Please wait while we load your content...