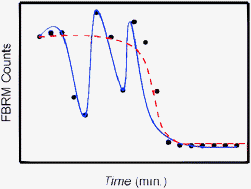
Oscillatory kinetic behavior is reported for the first time in a solid-state phase transformation of an organic compound, specifically, in the dissolution of Form V crystals of etoricoxib in neat toluene. The dissolution involves kinetics of bistability due to the generation of a second polymorph, Form I, during the process. While an attempt is made to reconcile the periodic behavior with the oscillatory rate coefficient predicted by time-dependent Marcus theory, more successful simulations of the data are obtained using superimposed, elementary dispersive kinetic models for both dissolution and nucleation-and-growth. Contrastingly, the dissolution of Form V in toluene containing a suspected small quantity of methanol/base contaminant shows that the phase transformation of Form V to the less-stable Form I crystals is rate-limiting and that the process is well described by a single, elementary dispersive kinetic model (i.e., no oscillations are observed). The latter observation lends support to the bistability hypothesis and provides evidence for the existence of a “corollary” to Ostwald's rule of stages.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...