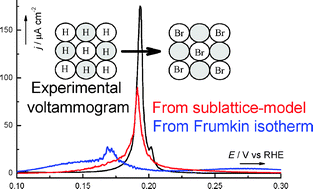
Previous work demonstrated that the Frumkin isotherm is inadequate to model the competitive coadsorption of species with different saturation coverages, such as hydrogen and bromide coadsorption on Pt(100) [N. Garcia-Araez et al., J. Electroanal. Chem., 2006, 588, 1]. Therefore, Monte Carlo simulations were necessary to determine meaningful values of the microscopic parameters (namely, energies of adsorption and interaction). In the present work, an alternative analytical isotherm is developed, by taking into account the occupation of two sublattices, which together compose the whole lattice of adsorption sites. Despite its relatively simple mathematical form, this isotherm presents, under certain conditions, a significant improvement over the classical Frumkin isotherm for the modeling of competitive adsorption processes, thus providing a closer agreement with results from Monte Carlo simulations. Finally, it is demonstrated that the sublattice-model isotherm will be generally applicable to systems in which the formation of segregated adlayers, whose structure is not explicitly taken into account in the model, is energetically unfavorable.

 Please wait while we load your content...
Please wait while we load your content...