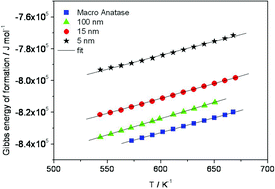
In view of increasing scientific and technological interest in nanomaterials, it is important to examine whether or, more exactly, to what extent the thermodynamic parameters change with size. Electrochemical e.m.f. measurements which provide a direct and elegant access to these thermodynamic data have been used in this study to investigate the excess contributions of anatase due to nano-size. The e.m.f. measurements are carried out (250–450 °C) on different particle sizes (1.2 μm–5 nm) using the cell: Au, O2, Na2Ti6O13, TiO2 (anatase) |Na-β′′ alumina |TiO2 (rutile), Na2Ti6O13, O2, Au. The e.m.f. observed is closely related to the difference of the Gibbs energies of formation (ΔfG°) of the titania crystals on both sides. Such cell voltage measurements with various sizes of anatase (1200, 100, 15, and 5 nm) as working electrodes enable us to calculate the excess enthalpy and entropy due to surface contributions and to provide refined data for the macroscopic anatase. No electrochemical Ostwald ripening or chemical Ostwald ripening was observed in the case of anatase nanoparticles up to 500 °C.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...