Effect of the intermolecular hydrogen bond conformation on the structure and reactivity of the p-cresol(H2O)(NH3) van der Waals complex
Abstract
The structure and reactivity of p-CrOH(NH3)2 and p-CrOH(H2O)(NH3) complexes were studied using mass-resolved one-colour resonance-enhanced multi-
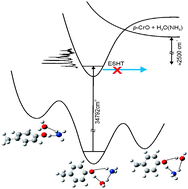
At the excitation energy of this work, the S1 state of p-CrOH(NH3)2 shows a sub-nanosecond lifetime, as determined by time-resolved
Substitution of NH3 by H2O closes the reaction
According to density functional theory calculations, the most stable isomer of the p-CrOH(H2O)(NH3) complex is a cyclic structure, in which H2O acts as the

 Please wait while we load your content...
Please wait while we load your content...