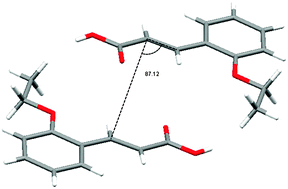
The photoreaction of two α-cinnamic acid derivatives, α-o-methoxy and α-o-ethoxy cinnamic acid, was studied by 13C CPMAS solid-state NMR spectroscopy in order to elucidate effects of aromatic substitution and substituent size on the kinetics of the [2+2] photodimerization. The reactants and products can be clearly differentiated and a detailed spectroscopic characterization was carried out, including 2D PASS spectra, at a low spinning frequency to determine the principal values of the chemical shift tensor. Density functional theory (DFT) calculations of chemical shifts and chemical shift anisotropy tensors were found to be in good agreement with the experimental results and helped in the individual assignments of reactant and photoproduct carbon atoms. The photoreaction kinetics show no systematic variation with substituent size, in that the α-o-methoxy cinnamic acid progresses at a slower rate than unsubstituted α-cinnamic acid, but α-o-ethoxy cinnamic acid at a faster one. Interestingly, the distance between reacting double bonds is not a good indicator of photoreaction rate. The observed trend is in part due to a larger degree of reorientation of the aromatic ring for the o-methoxy cinnamic acid, and a more dominant interaction appears to be the p-orbital overlap between two reacting double bonds in determining the reaction kinetics.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...