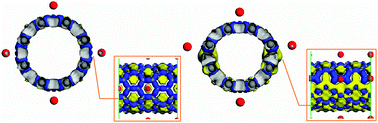
Implementation of hydrogen storage systems requires moderate bonding strength. However, this goal has remained a challenge, either due to the weak physisorption or extremely strong chemisorption. Here, we report on a new phenomenon, namely that H2 binding can be externally enhanced (or weakened) via superimposition of a positive (or negative) electric field. We demonstrate this concept using an 8-Li-doped carbon nanotube. The calculated adsorption energy Ead = −0.58 eV/H2 under F = +0.010 au is 93.33% lower than that under 0.000 au (F indicates the field intensity). This is because the positive field produces an extra dipole moment. In contrast, Ead increases from −0.30 to −0.20 eV/H2 when F = −0.010 au. In view of the fact that storage systems are insensitive to small unexpected field fluctuations, the application of the electric field as a reversible switch makes practical sense.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...