Frequency of negative differential resistance electrochemical oscillators: theory and experiments
Abstract
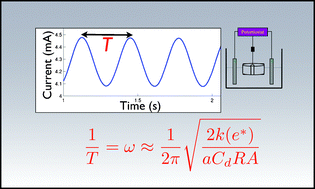
An approximate formula for the frequency of oscillations is theoretically derived for skeleton models for electrochemical systems exhibiting negative differential resistance (NDR) under conditions close to supercritical Hopf bifurcation points. The theoretically predicted ω∝ (k/R)1/2 relationship (where R is the series resistance of the cell and k is the rate constant of the charge transfer process) was confirmed in experiments with copper and nickel electrodissolution. The experimentally observed Arrhenius-type dependence of frequency on temperature can also be explained with the frequency equation. The experimental validity of the frequency equation indicates that ‘apparent’ rate constants can be extracted from frequency measurements of electrochemical oscillations; such method can aid future modeling of complex responses of electrochemical cells.

 Please wait while we load your content...
Please wait while we load your content...