Modified virtual orbitals for CI calculations of energy splitting in organic diradicals†
Abstract
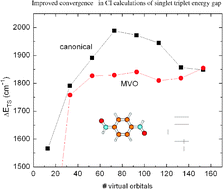
We have investigated the possible computational advantages of using modified virtual orbitals in CI calculations of singlet–triplet energy gaps in diradical systems, where the magnetic moieties are bridged by organic unsaturated fragments. The addition to the Fock operator of supplementary positive point charges onto the atoms sharing the unpaired electron in each of the two moieties provides a simple yet very effective recipe for the construction of modified virtual orbitals (MVOs) with significantly improved convergence to the limiting value of the singlet–triplet splitting. This is verified for all the systems investigated in the present study and paves the route towards the fully ab initio computation of magnetic couplings in large systems of current technological and/or biological relevance by supplementary/charge MVOs coupled to the localization and removal of orbitals centered far apart the active moieties, as proposed in a previous study.
- This article is part of the themed collection: Celebrating the centenary of the Italian Chemical Society

 Please wait while we load your content...
Please wait while we load your content...