Temperature dependence of the Oregonator model for the Belousov-Zhabotinsky reaction
Abstract
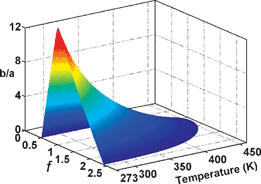
It is well known that temperature plays an important role in the chemical dynamics of Belousov-Zhabotinsky (BZ) reactions. The five-step Oregonator model of the BZ reaction has been elaborated here to investigate the temperature effect. The bifurcation dynamics has been calculated in the phase space spanned by initial reagents concentration ratio, stoichiometric factor and temperature. The combination of

 Please wait while we load your content...
Please wait while we load your content...