Structuring molecular hydrogen around ionic dopants: Li+cations in small pH2 clusters
Abstract
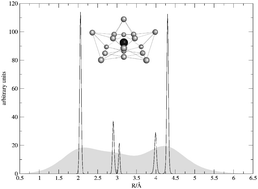
The formation of clusters of molecular hydrogen around a cationic charge, the Li+ ion, is modelled by treating the global interaction as a sum of potentials where the Li+–H2 forces come from a full anisotropic potential energy surface produced earlier in our group. The H2–H2 interaction is taken from the literature and treated as a spherical potential between structureless bosonic
- This article is part of the themed collection: Celebrating the centenary of the Italian Chemical Society

 Please wait while we load your content...
Please wait while we load your content...