Analysis of isotope effects in NMR one-bond indirect nuclear spin–spin coupling constants in terms of localized molecular orbitals
Abstract
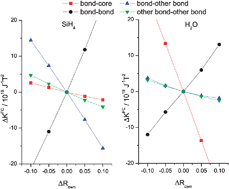
We recently showed, by analyzing contributions from localized molecular orbitals, that the anomalous deuterium isotope effect in the one-bond indirect nuclear spin–spin coupling constant of methane, also called the unexpected differential sensitivity, can be explained by the transfer of s-orbital character from the stretched bond to the other unchanged bonds [ChemPhysChem, 2008, 9, 1259]. We now extend this analysis of isotope effects to the molecules BH4−, NH4+, SiH4,

 Please wait while we load your content...
Please wait while we load your content...