Improvement of the capacitive performances for Co–Al layered double hydroxide by adding hexacyanoferrate into the electrolyte†
Abstract
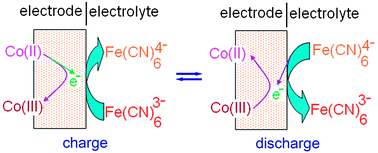
This paper reports on the improvement on the capacitive properties of Co–Al layered double hydroxide (Co–Al LDH) by adding hexacyanoferrate(II) and (III) solely or jointly into 1 M

 Please wait while we load your content...
Please wait while we load your content...