Does spin–orbit coupling effect favor planar structures for small platinum clusters?†
Abstract
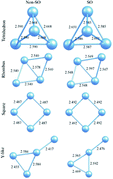
We have performed relativistic effective core potential calculations with and without spin–orbit coupling term in the framework of the density functional theory and investigated the geometry and binding energy of different isomers of free platinum clusters Ptn (n = 4–6) for the spin multiplicities from singlet to nonet. The spin–orbit coupling effect has been discussed for the minimum-energy structures, relative stabilities, vibrational frequencies, magnetic moments, and the highest occupied and lowest unoccupied molecular-orbital gaps. It is found in contrast to some of the previous calculations that 3-D configurations are still lowest energy structures of these clusters, although spin–orbit effect makes some planar or quasi-planar geometries more stable than some other 3-D isomers. Spin–orbit coupling effects change the relative stability of various isomers.

 Please wait while we load your content...
Please wait while we load your content...