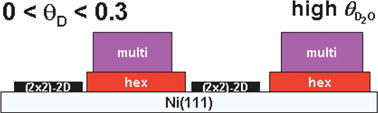
We have studied the surface coverage dependence of the co-adsorption of D and D2O on the Ni(111) surface under UHV conditions. We use detailed temperature-programmed desorption studies and high resolution electron energy loss spectroscopy to show how pre-covering the surface with various amounts of D affects adsorption and desorption of D2O. Our results show that the effects of co-adsorption are strongly dependent on D-coverage. In the deuterium pre-coverage range of 0–0.3 ML, adsorption of deuterium leaves a fraction of the available surface area bare for D2O adsorption, which shows no significant changes compared to adsorption on the bare surface. Our data indicate phase segregation of hydrogen and water into islands. At low post-coverages, D2O forms a two-phase system on the remaining bare surface that shows zero-order desorption kinetics. This two phase system likely consists of a 2-D solid phase of extended islands of hexamer rings and a 2-D water gas phase. Increasing the water post-dose leads at first to ‘freezing’ of the 2-D gas and is followed by formation of ordered, multilayered water islands in-between the deuterium islands. For deuterium pre-coverages between 0.3 and 0.5 ML, our data may be interpreted that the water hexamer ring structure, (D2O)6, required for the formation of an ordered multilayer, does not form anymore. Instead, more disordered linear and branched chains of water molecules grow in-between the extended, hydrophobic deuterium islands. These deuterium islands have a D-atom density in agreement with a (2×2)-2D structure. The disordered water structures adsorbed in-between form nucleation sites for growth of 3-D water structures. Loss of regular lateral hydrogen bonding and weakened interaction with the substrate reduces the binding energy of water significantly in this regime and results in lowering of the desorption temperature. At deuterium pre-coverages greater than 0.5 ML, the saturated (2×2)-2D structure mixes with (1×1)-1D patches. The mixed structures are also hydrophobic. On such surfaces, submonolayer doses of water lead to formation of 3-D water structures well before wetting the entire hydrogen-covered surface.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...