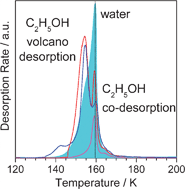
Despite considerable attention in the literature being given to the desorption behaviour of smaller volatiles, the thermal properties of complex organics, such as ethanol (C2H5OH), which are predicted to be formed within interstellar ices, have yet to be characterized. With this in mind, reflection absorption infrared spectroscopy (RAIRS) and temperature programmed desorption (TPD) have been used to probe the adsorption and desorption of C2H5OH deposited on top of water (H2O) films of various thicknesses grown on highly oriented pyrolytic graphite (HOPG) at 98 K. Unlike many other molecules detected within interstellar ices, C2H5OH has a comparable sublimation temperature to H2O and therefore gives rise to a complicated desorption profile. RAIRS and TPD show that C2H5OH is incorporated into the underlying ASW film during heating, due to a morphology change in both the C2H5OH and H2O ices. Desorption peaks assigned to C2H5OH co-desorption with amorphous, crystalline (CI) and hexagonal H2O-ice phases, in addition to C2H5OH multilayer desorption are observed in the TPD. When C2H5OH is deposited beneath ASW films, or is co-deposited as a mixture with H2O, complete co-desorption is observed, providing further evidence of thermally induced mixing between the ices. C2H5OH is also shown to modify the desorption of H2O at the ASW-CI phase transition. This behaviour has not been previously reported for more commonly studied volatiles found within astrophysical ices. These results are consistent with astronomical observations, which suggest that gas-phase C2H5OH is localized in hotter regions of the ISM, such as hot cores.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...