Aromaticity and magnetotropicity of dicyclopenta-fused polyacenes†
Abstract
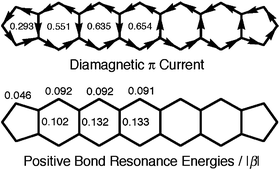
Most dicyclopenta-fused polyacenes are predicted to be moderately aromatic and diatropic, although they have no (4n + 2)-site conjugated circuits. We applied our graph theory of aromaticity and magnetotropicity to these molecules and found that these anomalous properties arise from a set of non-conjugated circuits, which contribute collectively to aromaticity and diatropicity. This result indicates that the conjugated circuit model is not always applicable to such non-alternant

 Please wait while we load your content...
Please wait while we load your content...