Performance of the DFT-D method, paired with the PCM implicit solvation model, for the computation of interaction energies of solvated complexes of biological interest
Abstract
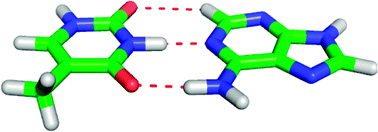
In this work we investigate the performance of the DFT method, augmented with an empirical dispersion function (DFT-D), paired with the PCM implicit solvation model, for the computation of noncovalent interaction energies of biologically-relevant, solvated model complexes. It is found that this method describes intermolecular interactions within

 Please wait while we load your content...
Please wait while we load your content...