Adsorption properties and vibrational spectra of propyne adsorbed on Rh(111). Comparison with other (111) metal surfaces†
Abstract
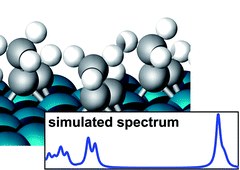
We have studied the adsorption properties of ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C axis parallel to a Rh–Rh bond and the molecular plane tilted away from the surface normal. The comparison between the adsorption behaviour of
C axis parallel to a Rh–Rh bond and the molecular plane tilted away from the surface normal. The comparison between the adsorption behaviour of

 Please wait while we load your content...
Please wait while we load your content...