Reactions of alkyl-radicals with gold and silver nanoparticles in aqueous solutions
Abstract
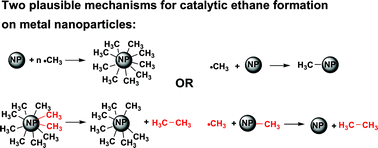
Silver and gold nanoparticles are very efficient catalysts for the dimerization of methyl-radicals in aqueous solutions. The rate constants for the reaction of methyl-radicals with the gold and silver nanoparticles were measured and found to be 3.7 × 108 M−1 s−1 and 1.4 × 109 M−1 s−1, respectively. The results thus suggest that alkyl-radicals, also not reducing ones, are scavenged by these nanoparticles. This might explain the role, if such a role exists, of these nanoparticles in medical applications.

 Please wait while we load your content...
Please wait while we load your content...