The kinetics and the mechanism of the reaction CH3C(O)O2
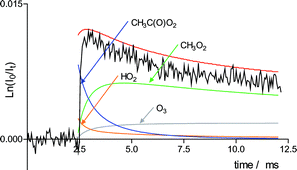
+ HO2 were reinvestigated at room temperature using two complementary approaches: one experimental, using flash photolysis/UV absorption technique and one theoretical, with quantum chemistry calculations performed using the density functional theory (DFT) method with the three-parameter hybrid functional B3LYP associated with the 6-31G(d,p) basis set. According to a recent paper reported by Hasson et al., [J. Phys. Chem., 2004, 108, 5979–5989] this reaction may proceed by three different channels: CH3C(O)O2
+ HO2
→ CH3C(O)OOH + O2 (1a); CH3C(O)O2
+ HO2
→ CH3C(O)OH + O3 (1b); CH3C(O)O2
+ HO2
→ CH3C(O)O + OH + O2 (1c). In experiments, CH3C(O)O2 and HO2 radicals were generated using Cl-initiated oxidation of acetaldehyde and methanol, respectively, in the presence of oxygen. The addition of amounts of benzene in the system, forming hydroxycyclohexadienyl radicals in the presence of OH, allowed us to answer that channel (1c) is <10%. The rate constant k1 of reaction (1) has been finally measured at (1.50 ± 0.08) × 10−11 cm3 molecule−1 s−1 at 298 K, after having considered the combination of all the possible values for the branching ratios k1a/k1,k1b/k1,k1c/k1 and has been compared to previous measurements. The branching ratio k1b/k1, determined by measuring ozone in situ, was found to be equal to (20 ± 1)%, a value consistent with the previous values reported in the literature. DFT calculations show that channel (1c) is also of minor importance: it was deduced unambiguously that the formation of CH3C(O)OOH + O2 (X 3Σ−g) is the dominant product channel, followed by the second channel (1b) leading to CH3C(O)OH and singlet O3 and, much less importantly, channel (1c) which corresponds to OH formation. These conclusions give a reliable explanation of the experimental observations of this work. In conclusion, the present study demonstrates that the CH3C(O)O2
+ HO2 is still predominantly a radical chain termination reaction in the tropospheric ozone chain formation processes.

 Please wait while we load your content...
Please wait while we load your content...