A universal empirical expression for the isotope surface exchange coefficients (k*) of acceptor-doped perovskite and fluorite oxides
Abstract
The isotope surface exchange coefficient k* determined in an 18O/16O exchange experiment characterises the exchange flux of the dynamic equilibrium between oxygen in the gas phase and oxygen in a solid oxide. At present there is no atomistic expression that relates measured exchange coefficients to materials’ parameters. In this study an empirical, atomistic expression is developed that describes the exchange kinetics of gaseous oxygen with diverse acceptor-doped perovskite and fluorite oxides at temperatures above T ≈ 900 K. The expression is used to explain the observed correlations between surface exchange coefficients k* and oxygen tracer diffusion coefficients D* and to identify compounds that exhibit high surface exchange coefficients.
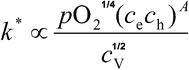

 Please wait while we load your content...
Please wait while we load your content...