Atomic orbitals in molecules: general electronegativity and improvement of Mulliken population analysis
Abstract
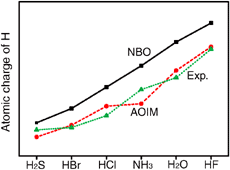
An approach of atomic orbitals in molecules (AOIM) has been developed to study the atomic properties in molecules, in which the molecular orbitals are expressed in terms of the optimized minimal atomic orbitals. The atomic electronegativities are calculated using Pauling’s electronegativity of free atom and are employed to find the electronegativity equilibrium in molecules and to describe the amphoteric properties of the transition metals from the groups 4 to 10. AOIM can also improve the numerical stability and accuracy of the original Mulliken population analysis.

 Please wait while we load your content...
Please wait while we load your content...